In April 2021, Kirk Humanitarian released a new version of UNIMMAP MMS that is now Halal-certified for use by Muslim women. The specifications below have been updated to reflect the new product.
Guidance to Governments, Program Developers, and Manufacturers
As governments, NGOs, and other partners look to transition from IFA to UNIMMAP MMS, there are several decision points to navigate, from formula through packaging.

Recommended Specifications
At Kirk Humanitarian, we support the manufacture of MMS consistent with the United Nations International Multiple Micronutrient Antenatal Preparation (UNIMMAP) formula, manufactured to international quality standards. UNIMMAP MMS is United States Pharmacopoeia (USP)-verified, which means it has been produced to meet rigorous quality and regulatory standards required of nutritional supplements marketed and sold in the United States. It is also Halal-certified by the Islamic Food and Nutrition Council of America (IFANCA).
Kirk Humanitarian’s UNIMMAP MMS tablets are categorized under the U.S. Food and Drug Administration (USFDA) regulations as a dietary supplement, delivered in a high-density polyethylene (HDPE) 180-count, tamper-evident, and child-resistant bottle with a 1-gram desiccant canister and carrying a 30-month expiration label claim under Zone IVB climatic and humidity conditions. This format of 180-count bottles is the recommended format today, until additional research indicates otherwise.
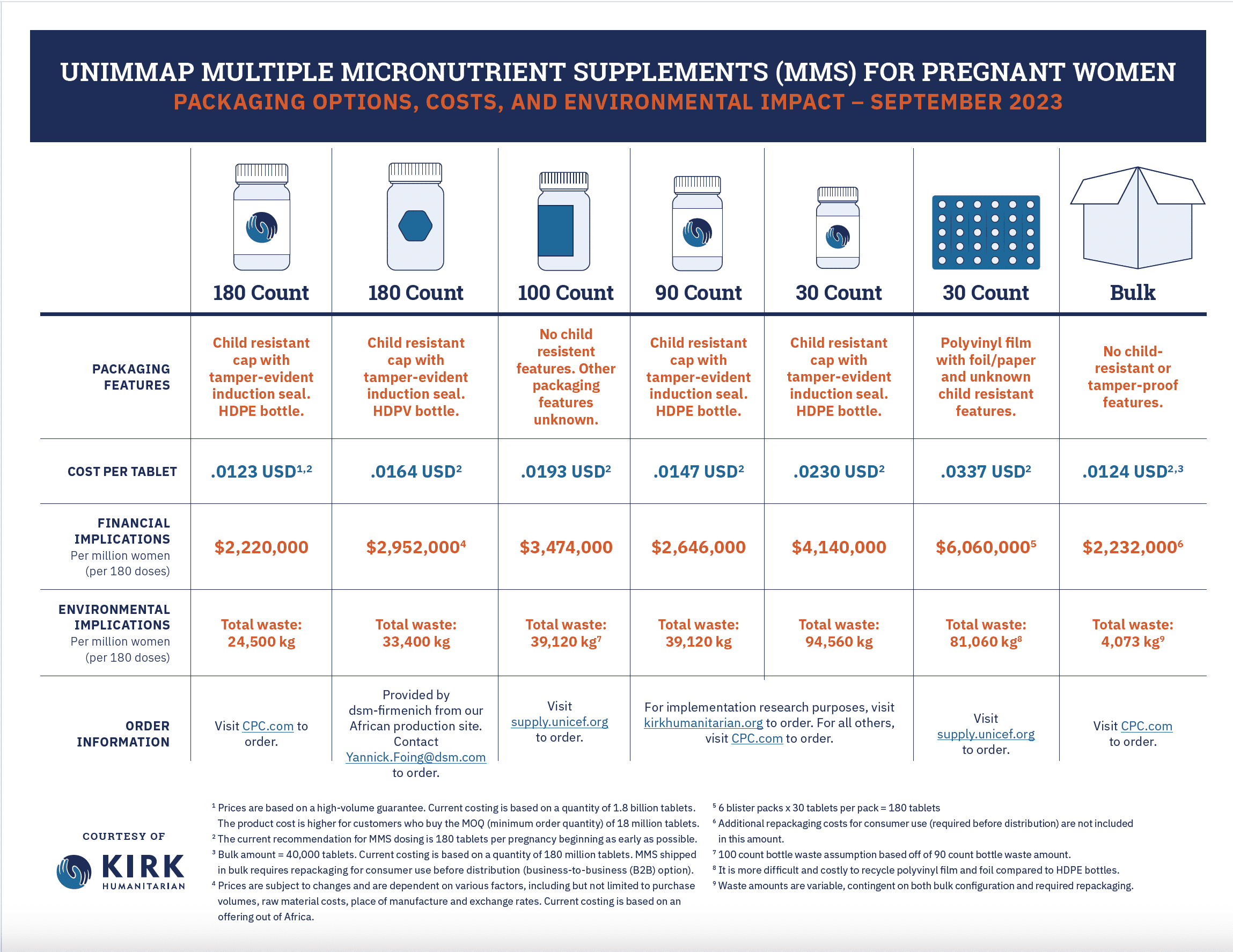
UNIMMAP MMS Packaging Options, Costs, and Environment Impact
UNIMMAP MMS is a superior product to IFA that can be — and is being — produced under optimized conditions of volume and high quality for about $2.22 USD per woman per pregnancy. At approximately $0.0123 per tablet, UNIMMAP MMS is produced by global suppliers at a price that is at or around cost parity with iron-folic acid (IFA). We have established pricing standards for 180-count bottles with global manufacturers of MMS, with various order sizes.


Our MMS Labeling, Packaging, and Supplementary Materials
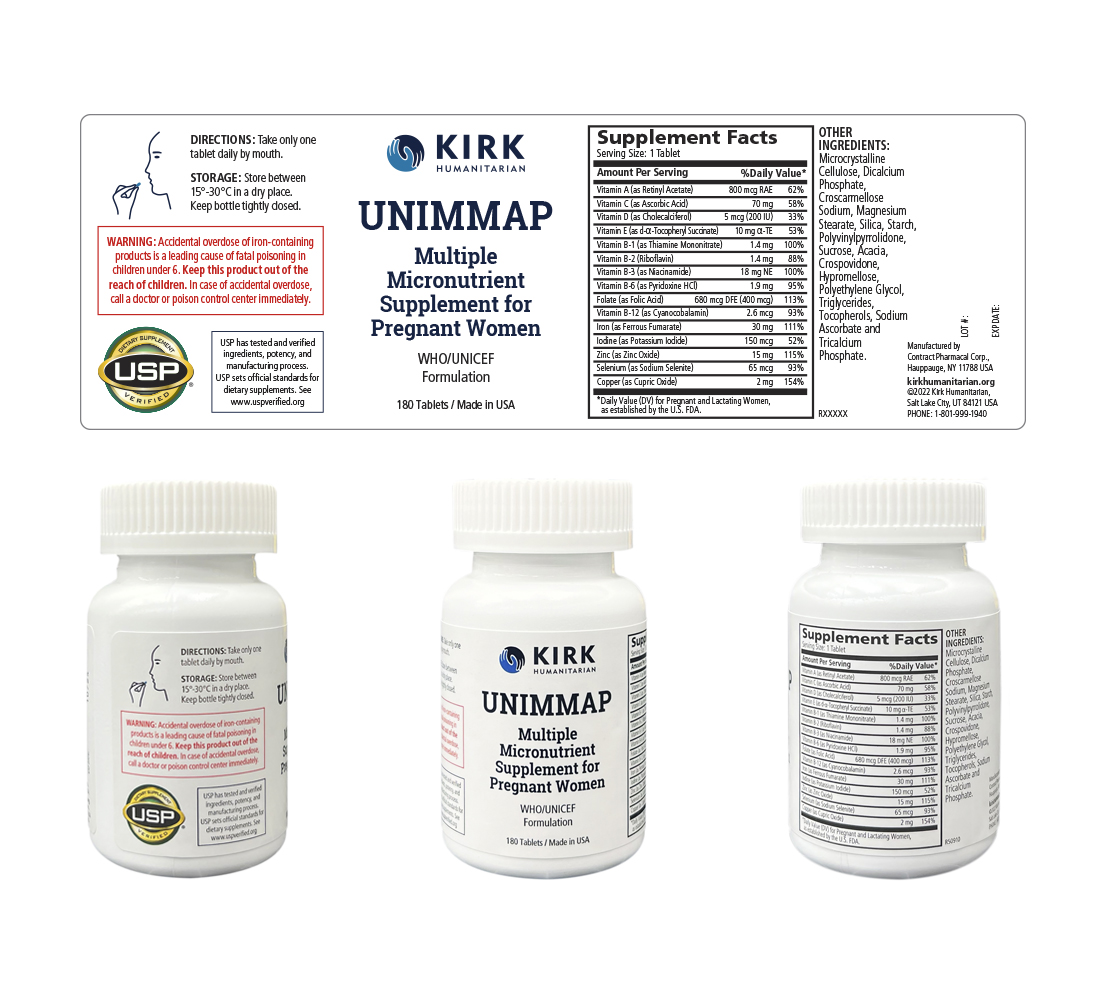
Label Information
- UNIMMAP MMS Product delivered by KH: oil- and water-soluble vitamins with minerals tablets
- USP-verified
- 30-month shelf life
- Zone IVB climatic conditions
- Dietary Supplement Panel

Bottle
- Tablets per bottle: 180
- Weight: 107 g
- Expiration: 30 months
- UV-resistant bottle
- Opaque (HDPE) material
- Tamper-evident
- Child–resistant
- Desiccant (1–gram canister)


Pallet
- Weight: 408 kg
- Boxes per pallet: 36
- Dimensions: 48″ x 40″ by 39.625″
Instructional Insert
A product usage and informational sheet for those distributing UNIMMAP MMS to pregnant women is included in each box and is intended to summarize the appropriate information that should be communicated to recipients of UNIMMAP MMS.
[MMS] is just one part of ensuring a positive pregnancy experience and healthy growth and development for all infants. But it’s something that can be done right now as policy–makers and health care providers strive for the best outcomes for women and children.”
Emily Smith, Harvard T.H. Chan School of Public Health


