UNIMMAP MMS Product Attributes
Together with our partners in maternal health and product manufacturing, we have identified six considerations when developing UNIMMAP MMS that, combined, deliver the best results for pregnant women and their babies.
UNIMMAP MMS should be:
Our Label, Packaging, and Materials
Product information for UNIMMAP MMS purchased by Kirk Humanitarian:
• United States Pharmacopoeia (USP)-verified
• Halal-certified
• 30-month shelf life
• Zone IVB climatic conditions
• Dietary Supplement Panel
Download the product label here.

Bottle
- Tablets per bottle: 180
- Weight: 107 g
- Expiration: 30 months
- UV-resistant bottle
- Opaque (HDPE) material
- Tamper-evident seal
- Child-resistant cap
- Desiccant (1-gram canister)


Box
- Weight: 10.7 kg
- Bottles per box: 90
- Includes instructional insert


Pallet
- Weight: 408 kg
- Boxes per pallet: 36
- Dimensions: 48 inches x 40 inches x 39.625 inches
How to Give and Use UNIMMAP MMS
Included in each box is a product usage sheet containing information in English, French, Spanish, and Arabic for healthcare providers to communicate to pregnant women before they begin taking UNIMMAP MMS.
Product Specifications & Packaging
As governments, NGOs, and others introduce and scale up UNIMMAP MMS, there are several product-related decisions to consider, from ingredients to how it’s packaged.
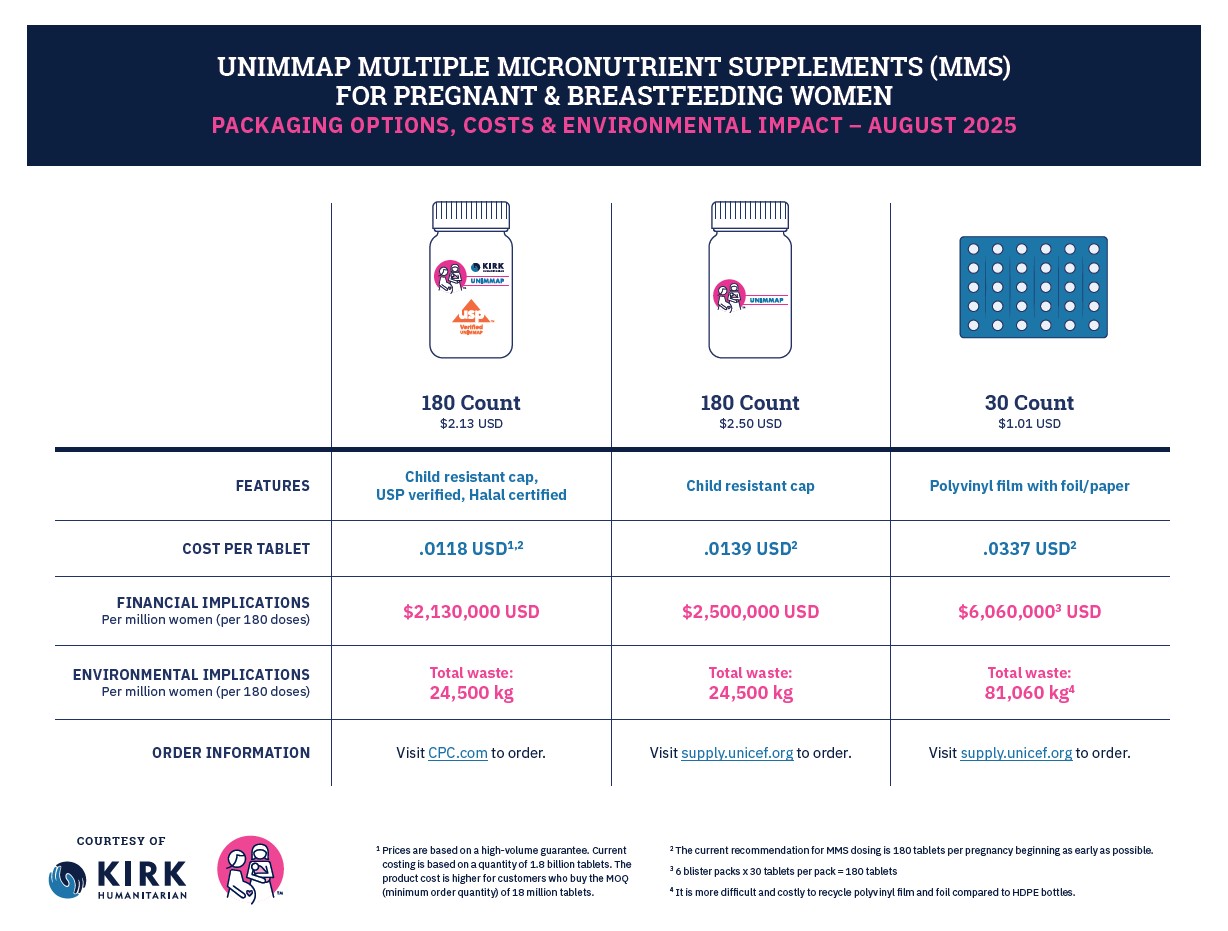
The UNIMMAP MMS that Kirk Humanitarian procures:
- Follows the WHO/UNICEF formulation that has consistently been proven more effective than other forms of MMS and IFA supplements.
- Meets rigorous quality and internationally accepted regulatory standards for dietary supplements. It has been verified by the United States Pharmacopeia (USP) and certified as halal by the American Halal Foundation (AHF) for use by Muslim women.
- Follows the rules of the U.S. Food and Drug Administration (USFDA) as a dietary supplement.
- Comes in a high-density polyethylene bottle with a child-resistant cap and tamper-evident seal. It’s packed with a 1-gram desiccant canister and carries a 30-month expiration label claim under Zone IVB climatic and humidity conditions. Each bottle contains 180 tablets, which is the recommended delivery format based on volume, to keep the cost per tablet low and comparable to IFA.
Read the detailed specifications for the UNIMMAP MMS Kirk Humanitarian procures by downloading them here.
Policy & Guidance
A strong global policy framework supports the use of MMS during pregnancy. Importantly, MMS is included in the World Health Organization’s Model List of Essential Medicines. This makes it easier for healthcare providers to use MMS in their services. Many countries have already included MMS in their own essential medicines list (EML), or they’re working to add it. To learn more about how to advocate for MMS to add to a country’s EML, click here.

Take a deep dive into MMS policy & guidance with these important resources.
- The World Health Organization has given specific advice on using MMS in emergencies since 2007. They also released recommendations for antenatal care in 2020.
- UNICEF has published guidance for countries on how to decide whether to use MMS in antenatal care. They’ve also provided programming guidance for maternal nutrition.
It’s important to note that while nearly 30 countries are currently integrating MMS into their health systems, many countries have not yet taken steps to transition. The superior maternal health and birth outcomes that MMS provides makes scaling up programs that deliver MMS to pregnant women an urgent opportunity.
For more MMS policy & guidance information, visit the Healthy Mothers Healthy Babies Knowledge Hub.